
Standard Operating Procedures - Vegetation Functional Traits Field Survey
KEY DETAILS
- Principal Investigator
- Dr. Nicola Stevens, Dr. Megha Ojha
Date - 1 June 2025
Version - 1.1.0
Programme - Rangelands Biodiversity Programme
Key partners - LCNR, Kew Gardens, University of Liverpool Contact email
- nicolastvns@gmail.com
1. PREAMBLE
Natural State’s research methods and activities are detailed by a set of accepted Standard Operating Procedures (SOPs). These documents describe the steps involved in all Natural State research methodologies, from data collection to data processing and storage. Each SOP documents key methodological details for a specific data type. The objectives and background of the projects or surveys these methods are used for, features of the study area where these methods are applied, and details on survey and sampling designs for these methods may be found in survey Design Documents (DDs), which are available in the Related Documents section below or may be perused in the main NS Design Documents documentation page.
2. GLOSSARY
3. METHODS OVERVIEW
A functional‐trait campaign standardizes measurement protocols to ensure data comparability and minimize observer bias. It targets representative sampling by focusing on species that drive ecosystem functions and using sufficient individuals and replicates to capture intraspecific variation. Sampling spans key habitat and environmental gradients and is integrated with established inventory plots for broader context. Traits are chosen for their clear mechanistic links to ecosystem processes, facilitating robust trait–environment analyses. Rigorous metadata recording, QR‐coded sample packaging, and standardized data capture ensure data quality and traceability. Finally, logistical efficiency and minimal ecosystem impact are achieved by confining destructive sampling to designated areas and maintaining controlled sample transport. Finally for this specific campaign we aim to introduce an extra layer of measurement to help tie remotely sensed data (drone and satellite) to on the ground field measurements, which is documented in the Aerial Cover Survey SOP. The Aerial Cover Survey should be completed before the Vegetation Functional Traits Field Survey as the aerial cover data is required to determine which species need to be sampled for functional traits.
3.1 METHOD AIMS
The Vegetation Functional Traits Field Survey is designed to:
- Target tree species that account for 80% of stem density and grass and forb species that account for 95% of aerial cover at designated sampling plots.
- Document plant level functional traits for 5-8 individuals per species through measurements taken in the field.
- Document leaf level functional traits for 3 leaves per individual through samples taken back to the lab.
3.2 METHODOLOGICAL BACKGROUND
The methodologies outlined in this SOP adhere to three primary trait-handbook sources to ensure standardization and comparability worldwide:
- Perez‑Harguindeguy, N., et al. (2016) Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 64(8):715–716.
- Cornelissen, J. H., et al. (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51(4):335–380.
4. SAMPLING PREPARATION
The equipment mentioned in the list below needs to be gathered, checked and packed before sampling begins. If any sensors need to be configured prior to commencing sampling this will be documented below the equipment list. Before any field sampling, verify and pack all required items.
4.1 EQUIPMENT LIST
- GEM plot QR codes
- Tablet with S123 installed
- Handheld GPS
- 6 X 50 m measuring tapes
- Zip lock/plastic bags
- Paper towel (to keep samples in zip locks damp)
- Large bottle of water for wetting paper towel
- QR code stickers
- Marker pen
- Meter rules (plant height)
- DBH tape
- Secateurs
- Small tape measure
- Metal rods (4mm diameter)
- Wood block
- Mallet
4.2 DEVICE CONFIGURATION
- The S123 app needs to be loaded onto the tablet and the Tree FT Field Survey, Forb FT Field Survey and Grass FT Field Survey forms need to be loaded.
- Station locations need to be loaded onto the handheld GPS.
5. SAMPLING PROCEDURES
A list of grass and forb species to sample will be created based on the Aerial Cover Survey (95 % of aerial cover). For each of these species, sample 5-8 individuals per species (to be reviewed). All plant level measurements should be done and recorded in the field. Following that, the measured individuals should be clipped as close to the base as possible and placed in a large plastic bag that is sealed and QR coded and transported back to the lab where leaf measurements will take place. All destructive sampling is to be done at outside the perimeter of the GEM plot.
Using plot-wide measurements from the GEM data on tree species >5 cm DBH, we have identified a subset of trees in each plot that cumulatively represent 80 % of stem density. Five individuals per species per plot should be sampled. Whole tree measurements should be done in the field, and then a small sunlight branch should be placed in a QR coded ziploc bag and taken back to the lab for leaf measurements. The branch should include at least 3 leaves. Additionally, 10 twigs < 10 cm in diameter should be collected per individual, placed into a QR coded ziploc bag and taken back to the lab. Lastly, one wood core needs to be taken per tree, placed into a QR coded ziploc bag and taken back to the lab.
A diagram of grass measurements is shown below: 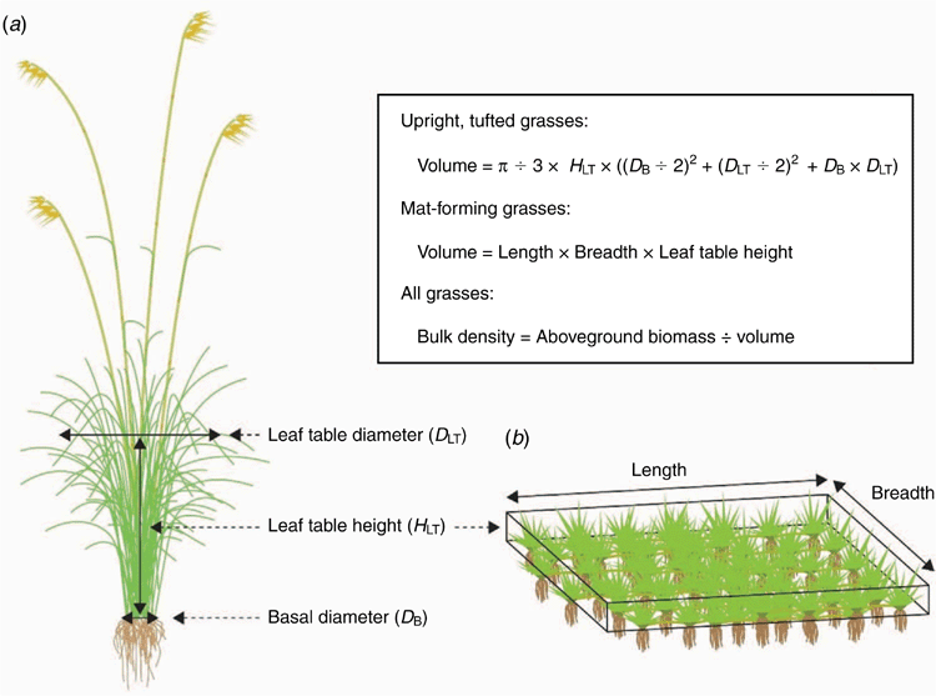
5.1 Grass functional trait collection
- Navigate to the plot.
- Establish the approximate extent of the 50 m X 50 m plot by running 50 m tapes along the southern and northern boundary to demarcate the outline of the plot. We are establishing this as we will be doing destructive sampling and we aim to do this sampling outside the plot unless absolutely necessary!
- Open the S123 App, and collect the metadata required by the Grass FT Field Survey Form.
- The plot coordinator will have a list of which tree, grass and forb species to collect. The day will start with everyone meeting to understand what species and number need to be collected from each growth form.
- Start with the first species on the list of grass species to sample at the plot you are in. Locate an individual of this species that is growing outside the plot. It should not have been recently grazed or senesced. On the same individual conduct the following measurements. 6.Culm diameter: Measure the culm diameter of the grass. Culm diameter is the maximum diameter of the second internode from the base of a grass culm. Identify the tallest flowering culm on each plant. Locate the second internode above the nearby node swelling and using calibrated digital calipers, measure the center of the internode, taking care to avoid the swelling itself. Be sure to include any leaf sheaths wrapped around the culm at the measurement site, since these sheaths increase effective bite thickness for grazers and contribute to fire insulation.
- Growth form: Identify the growth form of the grass: whether the grass is a tuft grass or a matt forming grass. If the grass is a tuft forming grass you will need to indicate whether it has stolons and whether it grows in tussocks.
- Bulk density: For tuft grasses, measure leaf table height, leaf table diameter and basal diameter. Leaf-table height is defined as the height visually estimated to correspond to the ~80th quantile of leaf biomass (the height below which the main bulk of the leaf canopy occurs). Measure leaf-table height for each plant using a measuring stick. Leaf table diameter is the diameter of the tuft at leaf table height. Basal diameter is the diameter of the base of the tuft.
- Bulk density: For matt forming grasses, mark out a square or rectangular section of grass matt and measure the leaf table height, length and breadth of this section.
- Sample Collection: Use secateurs to clip all above ground biomass from the base of the individual measured and place in a ziploc bag labelled with a QR code, the plot number and the species name. If it doesn’t fit into 1 bag put into multiple bags, label the bags appropriately (e.g. bag 1 of 3, bag 2 of 3, bag 3 of 3) and tape them together.
- A piece of wet paper towel should be placed in the ziploc bag with the plant material, and you need to blow into the ziploc bag before closing it to prevent the plant material from drying out. Keep in a cool shady location or cooler box.
- Repeat steps 5-11 for the rest of the individuals of this species.
- Repeat steps 5-12 for all other grass species on the list.
5.1 Forb functional trait collection
- Navigate to the plot.
- Establish the approximate extent of the 50 m X 50 m plot by running 50 m tapes along the southern and northern boundary to demarcate the outline of the plot. We are establishing this as we will be doing destructive sampling and we aim to do this sampling outside the plot unless absolutely necessary!
- Open the S123 App, and collect the metadata required by the Forb FT Field Survey Form.
- The plot coordinator will have a list of which tree, grass and forb species to collect. The day will start with everyone meeting to understand what species and number need to be collected from each growth form.
- Start with the first species on the list of forb species to sample at the plot you are in. Locate an individual of this species that is growing outside the plot. It should not have been recently grazed or senesced. On the same individual conduct the following measurements.
- Growth Form: Identify the growth form of the forb: whether the forb is creeping or erect.
- Plant Area: If the forb is a creeper, measure the length of the forb and the width of the forb. This is not required for erect forbs.
- Plant Height: Measure the plant height. Forb height is the vertical distance from the ground to the top of the vegetative part of the plant, excluding flowers.
- Spinescence: Record spinescence, which is the presence or absence of sharp, rigid structures (spines or prickles). Record presence (Yes) or absence (No) for each species based on visual inspection.
- Sample Collection: Use secateurs to clip all above ground biomass of the individual measured and place in a ziploc bag labelled with a QR code, the plot number and the species name. If it doesn’t fit into 1 bag put into multiple bags, label the bags appropriately (e.g. bag 1 of 3, bag 2 of 3, bag 3 of 3) and tape them together.
- A piece of wet paper towel should be placed in the ziplog bag with the plant material, and you need to blow into the ziploc bag before closing it to prevent the plant material from drying out.
- Repeat steps 5-11 for four more individuals of this species.
- Repeat steps 5-12 for all other forb species on the list.
5.1 Tree functional trait collection
- Navigate to the plot.
- Establish the approximate extent of the 50 m X 50 m plot by running 50 m tapes along the southern and northern boundary to demarcate the outline of the plot. We are establishing this as we will be doing destructive sampling and we aim to do this sampling outside the plot unless absolutely necessary!
- Open the S123 App, and collect the metadata required by the Tree FT Field Survey Form.
- The plot coordinator will have a list of which tree, grass and forb species to collect. The day will start with everyone meeting to understand what species and number need to be collected from each growth form.
- Start with the first species on the list of tree species to sample at the plot you are in. Choose a tree that is representative of the population and trees. To facilitate tree coring the trees must have a diameter of 10 cm or more at 1.3 m above the ground and be over 2 m in height. The tree should not be dead, pushed over, damaged or sick. The tree may be inside or outside the plot.
- Measure the tree height using a measuring stick or a hypsometer/clinometer.
- Measure the tree diameter at 30cm above the ground and at 130cm above the ground. Use a DBH tape for diameter measurements.
- Record spinescence, which is the presence or absence of sharp, rigid structures (spines or prickles). Record presence (Yes) or absence (No) for each species based on visual inspection.
- Use secateurs to clip a 30-50cm sunlit branch that is well vegetated and place in a ziploc bag labelled with a QR code, the plot number and the species name.
- A piece of wet paper towel should be placed in the ziplog bag with the plant material, and you need to blow into the ziploc bag before closing it to prevent the plant material from drying out.
- Use secateurs to clip to 10 small mature twigs (< 10 cm in diameter), and place these in a ziploc bag labelled with a QR code, the plot number and the species name. Add some wet paper towel and blow into the bag before sealing.
- Using an increment borer, extract a 5 cm long, 5 mm diameter core of wood at breast height (1.3 m) then immediately seal the sample in a ziploc bag containing wet paper towel to prevent moisture loss. Label with a QR code, the plot number and the species name. Use a thin metal rod to help extract the core from the corer.
- Repeat steps 5-12 for four more individuals of this species.
- Repeat steps 5-13 for all other tree species on the list.
6. POST PROCESSING
All plant samples should be brought back to the lab in blown up ziloc bags containing wet paper towel to prevent them from drying out.
6.1 SAMPLE PROCESSING AND STORAGE
Samples will be processed in the lab according to the Vegetation Functional Traits Lab Measurements SOP.
6.2 DATA ENTRY AND UPLOADS
S123 forms need to be sent to the cloud upon return to internet or cell phone reception.
7. RELATED DOCUMENTS
7.1 DESIGN DOCUMENTS
7.2 OTHER RELEVANT SOPS
7.3 DATA ELEMENTS
Survey Design
Data Collection
- S123 data collection form - Grass FT Field Survey
- S123 data collection form - Forb FT Field Survey
- S123 data collection form - Tree FT Field Survey
Dashboard
8. REVISION AND VERSION HISTORY AND DESCRIPTION
v1.0.0 Initial SOP created in May 2025. v1.1.0 Modifications after in person meeting on first day of trait campaign in June 2025.