
Rangelands invertebrate pitfall trap survey lab processing
KEY DETAILS
- Principal Investigator
- Bjoern Matthies
Date - 2 December 2024
Version - 0.1.0
Programme - Rangelands Biodiversity Project (RBP)
Study Site - Lewa-Lolldaiga-Borana-Ngare Ndare (LLBN)
Key partners - University of Liverpool
Contact email - b.matthies@liverpool.ac.uk; kate.parr@liverpool.ac.uk
1. PREAMBLE
Natural State’s objectives and activities are detailed by a set of accepted Standard Operating Procedures (SOPs). These documents describe the steps involved in all Natural State projects, from data collection to data processing and storage. Each SOP documents key project details and provides methodological details specific to the project. The objectives and background of the project, features of the study area, and details on survey and sampling design may be found in the project Design Document (DD) which is available in the Related Documents section below.
1.1 SOP PURPOSE
To provide a clear step by step guide to the methods implemented in the project, therefore allowing for consistency in data collection and repeatability of all steps involved in a project’s data collection, processing and storage. This is crucial to Natural State’s mission of facilitating nature restoration at scale by using the latest technology and methods to revolutionise impact monitoring for carbon, biodiversity and human well-being.
1.2 SOP SCOPE
This document details how this project will be implemented. All methodological steps are explained, and the principal team members overseeing the project are listed in case further clarification is required. It also further directs readers to where they can find additional information relevant to the project. This document is intended to be printed out and taken to the field for reference sake.
2. GLOSSARY
- BACI
- Before-After Control-Impact survey design
- CPP
- Carbon Pool Plot. A 50m X 50m sampling site within which NS measures aboveground and belowground carbon stocks.
- Deployment
- The period of time a single remote sensor is active within the environment at a single, defined station as part of a survey.
- GEM
- Global Ecological Monitoring. A global biological research project to measure ecological processes.
A 50m X 50m sampling site within which NS measures aboveground and belowground carbon stocks and rates of carbon cycling. - Pitfall Trap
- A round plastic container containing glycol that is buried approximately 10 cm deep and used to collect ground dwelling invertebrates.
- Project
- A concerted, data-driven effort to robustly measure variation in Biodiversity, Carbon, or Human-wellbeing in response to one or more sources of heterogeneity in a designated landscape.
- S123
- Survey123, a field-data collection app from ESRI which NS uses for recording all field observations and survey metadata.
- Sampling Protocol
- Explicit survey methodology that describes the design, effort, duration, configuration, and operation of a survey.
- Sampling Site
- A distinct, discrete spatial unit defined in at least two dimensions where sampling occurs.
- Station
- A point location where sampling occurs in space.
- Study Area
- A defined geographic region of interest within which one or more surveys investigate ecological patterns at one or more sites.
- Survey
- A set of simultaneous deployments of remote sensors over a defined period of time at a coordinated set of stations for the purposes of collecting data on the environment and its communities.
3. PROJECT OVERVIEW
3.1 PROJECT AIMS
The Rangelands Invertebrate Pitfall Trapping Survey aims to:
- Measure ground invertebrate biodiversity to contribute to the Rangelands Carbon-Biodiversity Survey.
- Measure the effect of vegetation structure on invertebrate diversity.
- Measure the effect of soil type on invertebrate biodiversity.
- Measure the effect of grazing type and intensity on invertebrate biodiversity.
3.2 PROJECT BACKGROUND
NATURAL STATE’s overarching goal is to catalyze big scale ecological restoration globally by revolutionizing impact monitoring, generating innovative, new, nature-based financial mechanisms and developing local leadership capacity. As part of this, NATURAL STATE is attempting to create impact monitoring systems that reduce the cost of measuring change in an environment, and that can provide traceable and verifiable results that can be used to inform financial instruments on the impact of interventions.
Invertebrate communities are an integral component of healthy ecosystems. Given the relatively short life cycles of many invertebrate taxa combined with their rapid progression through life stages, invertebrates are quick to respond to landscape level change and consequently often used as ecological indicators. Despite their small body size, invertebrates such as ants and termites account for the largest share of faunal biomass and act as ecosystem engineers, fundamentally impacting the structure and function of landscapes. They are essential for nutrient cycling and carbon sequestration. An in depth understanding of invertebrate communities therefore provides important insights into ecosystem health and is applicable to almost all landscapes, regardless of intervention type and management regime, which sometimes exclude larger taxa through the use of artificial barriers.
3.3 STUDY AREA
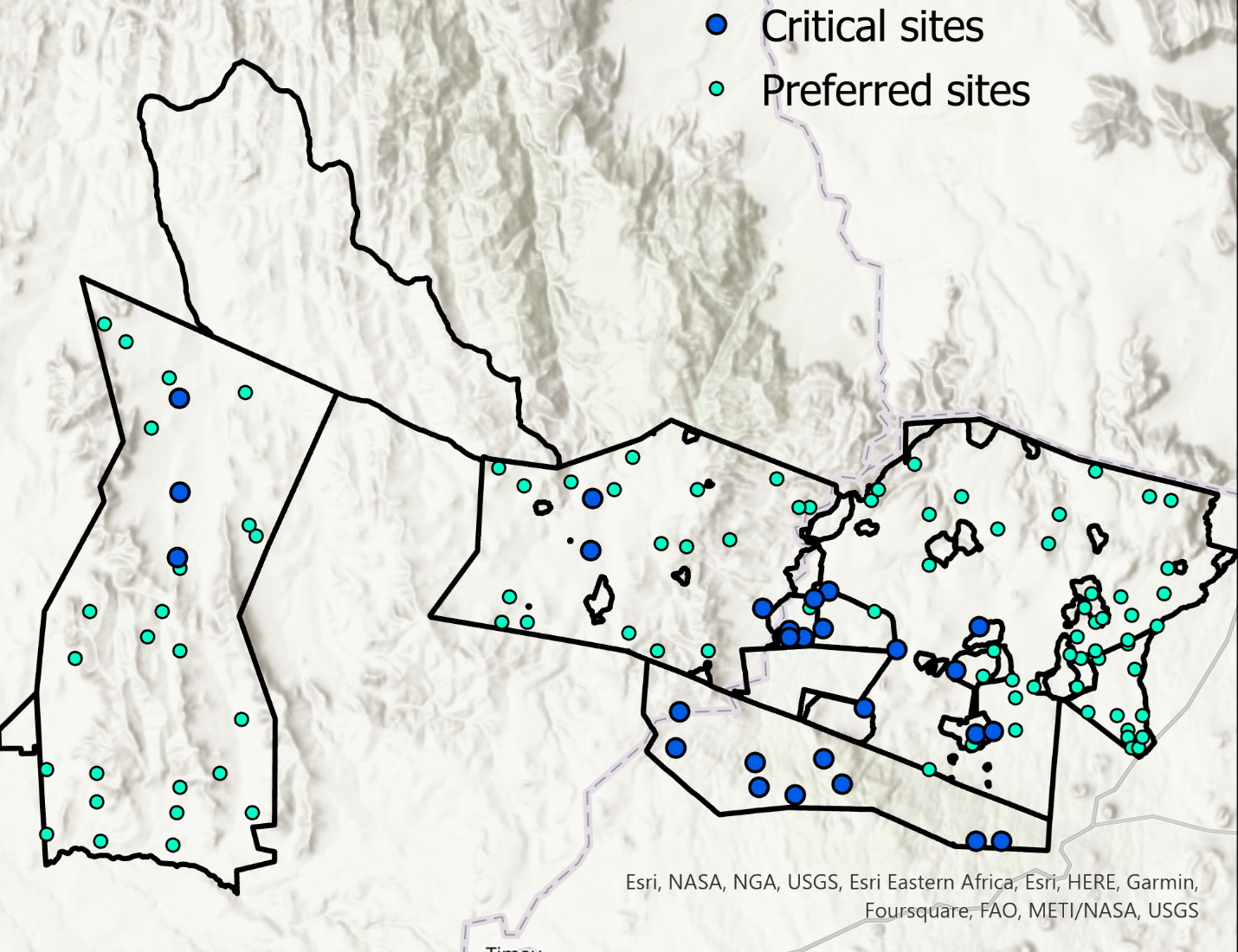
The Rangelands Invertebrate Pitfall Trapping Survey will be conducted across Lewa, Borana, and Lolldaiga conservancies. Sampling will occur at all GEM plots as well as a subset of carbon pool plots with variation in tree cover, soil type and grazing intensity. These will be a subset of plots at which the Carbon-Biodiversity survey takes place. The following is a map of the planned Carbon Biodiversity survey sampling sites.
3.4 PROJECT TIMELINE
The temporal design of the survey should track the temporal sampling from the corresponding carbon survey. Where long-term monitoring of carbon occurs (i.e., GEM plots) invertebrate monitoring can be replicated seasonally. Where carbon sampling is replicated over time (e.g., CP plots that are part of any exclosure BACI survey, usually where an intervention has been imposed), invertebrate sampling should occur whenever the carbon is sampled. Where carbon is only sampled once, invertebrate sampling should occur for a single deployment.
The Invertebrate survey will therefore be comprised of a number of sub-surveys:
Critical Sub-Surveys
- GEM wet 2023/2024 (October – April 2024): sampling at all GEM 18 GEM plots
- CPP dry 2024 (May/June - July 2024): sampling at a subset of CPP plots, specifics still to be determined
Ideally, all invertebrate sampling should be done during the wet season or soon afterwards, as invertebrate activity in the dry season will be low. Invertebrate sampling during or at the end of a long dry season should be avoided.
Sub-surveys are subject to change as the NS carbon sampling efforts and resources evolve. Additional sub-surveys will be added on in due course.
4. SAMPLING PREPERATION
The equipment mentioned in the list below needs to be gathered for use during lab processing.
4.1 EQUIPMENT LIST
- Phone with S123
- 300ml pitfall containers with samples
- 1 empty pitfall container
- 50ml plastic tubes with QR codes stuck on
- 2ml plastic tubes for ants
- Empty plastic bottle to recapture the fixative (propylene glycol)
- 70% Ethanol
- Forceps
- Small sieve
- Funnel
- Datasheet for Invertebrate orders (currently in Excel or S123 form still to come)
- Microscope
- Identification keys
- Petri dishes
- Scale
- Paper
- Pencil
4.2 DEVICE/EQUIPMENT CONFIGURATION
Ensure each 50ml tube has a QR code stuck onto it.
5. SAMPLING PROCEDURES
This protocol details the lab procedures for processing invertebrate pitfall trap samples. There are 4 steps required, and an optional 5th step for calibratating bulk weitghts. This step should be ignored unless specifically requested by the project PI.
5.1 CHECK SAMPLES INTO LAB
- Samples will arrive in a 300ml plastic container with a QR code attached to the bottom.
- When they arrive in the lab, they have to be scanned and registered with the S123-form “Lab Sample Log”. At this stage, the containers are usually filled by a mix of invertebrates, propylene-glycol and often soil or grasses or leaves.
5.2 WASH SAMPLES AND TRANSFER INTO NEW CONTAINERS WITH NEW QR CODES
In this step, the samples get washed to separate them from the propylene glycol and to clean them from soil and transferred into smaller (50ml) plastic tubes which are filled with ethanol. There are a number of steps to go through this process:
- Sample exchange:
a. Attach a QR code to the 50ml tube if not present already.
b. Use the sample-transfer form to scan a 300ml pitfall cup and then a 50ml tube. - Washing the sample:
a. Attach the funnel to the empty plastic bottle
b. Pour the sample into the sieve, above the funnel so that the fixative is getting collected in the bottle.
c. After the fixative has been collected, remove the bottle and funnel and gently rinse the samples in the sieve under the running tab (on low water pressure) to remove soil or remaining fixative from the invertebrates.
d. Put the sieve upside down on an empty pitfall container and flick with your finger against to push the invertebrates into the container.
e. Use a blunt forceps to collect remaining invertebrates that are stuck to the sieve and put them in the container too.
f. Add a small amount (15-30ml) of 70% Ethanol to the container.
g. Pour the content of the container into the previously scanned 50ml tube, use forceps again to transfer any remaining invertebrates. Make sure that there is enough Ethanol to cover all invertebrates. If there are very large individuals or a large amount of invertebrates, fill up the tube completely.
5.3 IDENTIFY SAMPLES UNDER THE MICROSCOPE
In this step, the collected invertebrates will be counted and identified to order level. Ant specimens will be stored in separate containers. After identifying the invertebrates from each individual trap, all non-ant invertebrates from the 15 pitfall traps from one sampling site will be combined together (“Bulk sample”):
- Read the label and record in data sheet. In future this will be done by scanning the QR code of the container into an S123 form.
- Empty the sample tube onto a petri dish.
- Place the petri dish under the stereo microscope.
- Use the identification keys to identify the invertebrates to order level, and count how many specimens per order are present in the sample. Enter this information into the datasheet. In the future this will be replaced by an S123 form.
- Transfer the ant specimens from this sample to a 2ml tube and add a label (pencil writing of Sample ID on paper put inside the tube).
- After identifying, transfer all other invertebrate specimens into the Bulk sample tube. This should be a 50ml tube with a QR code. The S123 form for scaning the QR code is still being developped. A pencil on paper label with site ID should be included in the tube with the sample.
5.4 RECORD BULK WEIGHT
After all 15 pitfall traps from one site have been combined into a bulk sample (without ants), their weight should be recorded:
- Empty the bulk samples into a sieve to get rid of the ethanol and then empty the samples into a petri dish.
- Leave the dish uncovered and allow the remaining ethanol evaporate for 1 hour.
- After that 1 hour, use an empty petri dish to calibrate the scale.
- Replace the empty petri dish with the one filled with the samples and record its weight in the datasheet.
- Afterwards, return the invertebrate samples to their 50ml tube and refill the tube with ethanol.
5.5 BULK WEIGHT CALIBRATION (OPTIONAL)
This step is performed to collect calibration data, only when requested by the project PI. Otherwise it should be ignored:
- After recording the wet weight put the petri dish containing the invertebrates into a drying oven.
- Dry at 60 degrees Celcius for 72 hours.
- After 72 hours use an empty petri dish to calibrate the scale.
- Weigh the dried invertebrate sample and record the weight in the datasheet.
6. POST PROCESSING
This section details all steps that need to be followed after the lab processing is complete to ensure the samples are properly stored.
6.1 SAMPLE PROCESSING AND STORAGE
Store the bulk samples in a plastic container with the lid on to prevent ethanol evaporation. Check samples every 3 months and refill with ethanol if necessary.
Put all 15 ant samples from a site together in a single ziploc bag. Place this ziploc bag into a plastic container with the lid on to prevent evaporation. These will be exported Liverpool.
6.2 DATA ENTRY AND UPLOADS
All data entry for this project currently happens in an excel spreadsheet called [2024.11.14]_pitfall_trap_datasheet.xlsx. A new documet with an updated date should be saved every day.
7. RELATED DOCUMENTS
7.1 DESIGN DOCUMENT
[In Progress]
7.2 OTHER RELEVANT SOPS
7.3 DATA ELEMENTS
Survey Design
Data Collection
8. REVISION AND VERSION HISTORY AND DESCRIPTION
==XXX==
9. SIGNATURES OF CONFIRMATION
Principal Investigator: ______________ Date: ___________
Director of Impact Insights: ____________ Date: ___________
10. APPENDICES
None currently available